Professor Sandra Schmid – The Dynamics of Dynamin and Cancer Evolution
All cells need to transport vital biomolecules across membranes by packaging them into small membrane-bound containers called vesicles. Integral to this process is the large protein dynamin. In her insightful and pioneering research, Professor Sandra Schmid has begun to open the ‘black box’ on how changes in the activity of dynamin can impact cargo uptake to vesicles and the survival of cancer cells.
Membrane Fission and Fusion and Cellular Communication
To separate their internal from their external environment, living cells have a ‘skin’, called the plasma membrane, that consists of an impenetrable double layer of lipids. The main function of the plasma membrane is to protect the cell from its outside environment – however, proteins embedded in the plasma membrane serve as border guards to control the movement of substances in and out of cells, as well as facilitate communication between cells and their environment.
In addition to the plasma membrane animal and human (eukaryotic) cells have many membrane-bound structures within them called organelles, which are specialised compartments with specific functions. For example, the endoplasmic reticulum, Golgi complex and lysosomes are organelles involved in the manufacture, export and degradation of proteins, respectively.
While the various organelles in eukaryotic cells are clearly distinct, they are not static in composition. How, then, are the different organelles made and maintained? The answer is that proteins are synthesised and packaged into membrane vesicles and targeted to their intracellular destinations based on sorting signals within the proteins themselves and on the vesicles.
This process, known as vesicular trafficking, is highly regulated and critically depends on membrane fission and fusion. Membrane fission is the formation of two separate membranes from a single one while fusion is the opposite, the merging of two separate membranes into a single membrane.
The Protein Machinery Behind Membrane Fission
The process and proteins involved in membrane fusion have been extensively characterised, as recognised by the 2013 Nobel Prize in Physiology or Medicine. In contrast, much less is known about the cellular mechanisms behind membrane fission, except as it occurs during clathrin-mediated endocytosis, the major pathway for vesicle formation from the plasma membrane.
‘We worked for over 20 years on dynamin and its role in fission and now find that it is also pivotal in regulating early stages of clathrin-mediated endocytosis. This is the most exciting to me, as we know little about how dynamin does this.’
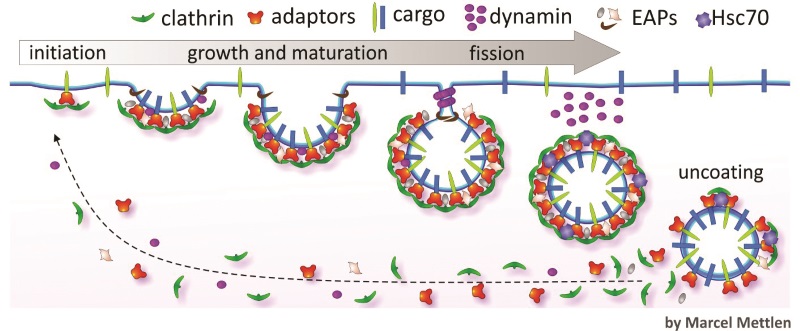
As inferred by its name, clathrin-mediated endocytosis involves the protein, clathrin, which assembles into basket-like lattices that coat small regions of the plasma membrane to drive vesicle formation. Clathrin-mediated endocytosis is initiated when there is a cargo that contains specific protein sequences or motifs that act as internalisation signals. These motifs are recognised by adaptor proteins that recruit clathrin to form new clathrin-coated pits.As clathrin assembles, the clathrin-coated pits undergo maturation until they form deeply invaginated buds still attached by a narrow neck that await membrane fission to release the vesicle containing the ingested material for distribution within the cell.
Membrane fission is driven by dynamin, another protein critical for clathrin-mediated endocytosis. Dynamin assembles around the narrow necks of deeply invaginated coated pits and with the aid of several other accessory proteins, catalyses membrane fission and the ‘budding off’ of vesicles.
The Relentless Pursuit to Understand Dynamin Function
Recognising the fundamental need to understand how dynamin works, Professor Sandra Schmid and her group have studied the role of dynamin in membrane fission and clathrin-mediated endocytosis for over 20 years. She explains that, ‘clathrin-mediated endocytosis is the major pathway by which cells take up nutrients and signaling hormones and it also plays a critical role in controlling inter-cellular communication and signaling.’
Schmid obtained her PhD at Stanford University and was a postdoctoral fellow at Yale University before becoming an Assistant Professor at The Scripps Research Institute in 1988. In 2012, she was recruited to the University of Texas Southwestern Medical Center, where she holds the Cecil H. Green Distinguished Professor of Cellular and Molecular Biology.
Throughout her career she has worked to understand the role of dynamin in endocytosis and her team’s ground-breaking work demonstrated for the first time the pivotal role of dynamin in membrane fission. Schmid describes how, ‘we worked for over 20 years on dynamin and its role in fission and now find that it is also pivotal in regulating early stages of clathrin-mediated endocytosis. This is the most exciting to me, as we know little about how dynamin does this.’
Currently, her laboratory focuses on understanding the regulation of clathrin-mediated endocytosis in normal and cancerous cells. One exciting line of this research suggests that evolving cancer cells can qualitatively and quantitatively alter endocytosis and vesicular trafficking, a phenomenon she has termed ‘adaptive’ clathrin-mediated endocytosis. Schmid describes how, ‘we have recently discovered that some cancer cells alter their clathrin-mediated endocytosis machinery in ways that can enhance their survival and migration.’
Dynamin and Cell Signalling
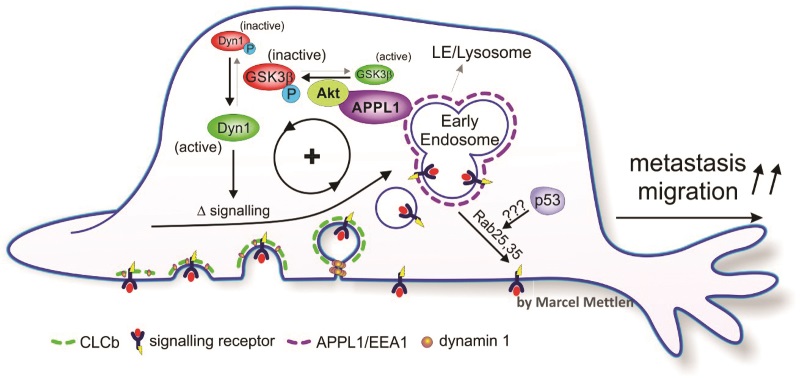
Dynamin is known to exist in three forms in mammals: dynamin-1 (Dyn1), known to be present at very high levels in neurons in the brain; dynamin-2 (Dyn2), present at lower levels in all cell types; and dynamin-3, which is present in testis, brain and lung. The three forms are nearly identical, but recent studies from the Schmid lab have shown that they can differentially regulate clathrin-mediated endocytosis.
Dyn1 was presumed to be specific to neurons, but the levels of both Dyn1 and Dyn2 are, in fact, similar in most other tissues. Schmid’s team confirmed that Dyn1 is present but normally inactive in non-neuronal cells; however, it can be activated when needed for rapid clathrin-mediated endocytosis. They also observed that many cancer cells express elevated levels of Dyn1, which motivated their work on adaptive clathrin mediated endocytosis.
Dyn1 is highly regulated by a process called phosphorylation. This process involves modification of a protein by addition of a charged molecule containing phosphate that acts to control the activity of a protein or its binding to others. The proteins GSK3β and Akt are known to be involved in the phosphorylation of Dyn1 and act in opposition to control its activity: GSK3b inhibits Dyn1 activity and Akt inhibits GSK3b, thus increasing Dyn1 activity. Importantly, Akt is also frequently activated in cancer cells.
Schmid’s team investigated whether the phosphorylation of Akt/GSK3β may be responsible for the activation of Dyn1 in cancer cells with altered clathrin-mediated endocytosis. They found that the rate of clathrin-mediated endocytosis was increased when GSK3β was inhibited, and decreased when Akt is inhibited. These effects were abolished when Dyn1 was eliminated by genetic modification. The team showed that more clathrin-coated pits assemble and the time it takes for them to mature decreases with Dyn1 is activated.
The team concluded that enhanced Akt/GSK3β signalling activates Dyn1, leading to increased rates of maturation of clathrin-coated pits and abnormal clathrin-mediated endocytosis in cancer cells.
How is Dynamin Exploited by Advancing Cancer Cells?
Schmid’s team is investigating whether the altered or ‘adaptive’ clathrin-mediated endocytosis in cancer cells may drive cancer progression. She states that, ‘my lab is working to identify the components of the clathrin-mediated endocytosis machinery that regulate the process to define the mechanisms underlying this regulation in both normal and cancer cells.’
Clathrin is composed of three clathrin heavy chains and three clathrin light chains. Humans and animals express two forms of clathrin light chains, CLCa and CLCb, with unknown functional differences. Interestingly, CLCb is preferentially increased in non-small-cell lung cancer, the cancer type that accounts for the great majority of all lung cancers. Moreover, aggressive lung tumours express more CLCb than early stage tumors.
Therefore, to understand the functional differences between CLCa and CLCb in clathrin-mediated endocytosis, Professor Schmid and her group generated cells where CLCb is the predominant form and CLCa is reduced, referred to as ‘switched’ CLCb cells.
Since altered signalling by epidermal growth factor receptors contributes to cancer progression, the Schmid lab examined epidermal growth factor receptor signalling, endocytosis and recycling in switched CLCb cells. Treatment with epidermal growth factor, which activates the receptor, prompted prolonged and stronger Akt/GSK3β phosphorylation, leading to increased Dyn1 activation and higher rates of receptor endocytosis and recycling. The accelerated rate of receptor endocytosis and recycling is dependent on Dyn1, as this effect was abolished when Dyn1 was depleted.
These changes in endocytic trafficking led to an increase in migration speed in switched CLCb cells. In mice, the spread of a tumour to distant sites (metastasis) was two times higher for switched CLCb cells. Furthermore, lung cancer patients with relatively high levels of CLCb or Dyn1 had significantly worse survival rates than those with low levels of expression.
Taken together, the team’s findings indicate that epidermal growth factor receptor signalling activates Dyn1 through the Akt/GSK3β pathway, to change epidermal growth factor receptor endocytosis and recycling in switched CLCb cells. This change impacts on cancer migration, the spread of tumours and patient survival.
Activated Dynamin Helps Cancer Cells Evade Programmed Cell Death
As an anticancer strategy, the protein TRAIL (TNF-related apoptosis-inducing ligand) that binds to death receptors and activates programmed cell death, or apoptosis, has shown some potential. However, the contribution of clathrin-mediated endocytosis and dynamin to the regulation of TRAIL and death receptor programmed cell death is unclear.
Remarkably, Schmid and her team observed that Dyn1-depleted human breast cancer and lung cancer cells are more sensitive to cell death after treatment by TRAIL. TRAIL and death receptor endocytosis was also strongly inhibited in these cells. When Dyn1 was restored, both TRAIL and death receptor endocytosis was re-established along with TRAIL resistance. Dyn1 can be activated by calcineurin, a calcium dependent enzyme that removes phosphate. When calcineurin is inhibited the sensitivity of cells to TRAIL-induced cell death significantly increased.
This suggests that further understanding of TRAIL and death receptor endocytosis, that allows cancer cells to internalise death receptors to avoid programmed cell death, could be important for future research into anticancer treatment strategies.
CREDIT: Marcel Mettlen
Future Perspectives
Schmid and her team have been instrumental in uncovering a previously unsuspected role for Dyn1 and adaptive clathrin-mediated endocytosis in cancer proliferation, migration and the spread of tumours.
Yet, these findings also lead to many other unanswered questions. For example, how common is adaptive clathrin-mediated endocytosis in cancer? Are there other signalling pathways that can alter this process? What are the mechanisms that drive the specific differences in function of the different forms of dynamin, such as Dyn1’s acceleration of clathrin-coated pit maturation?
Given that adaptive clathrin-mediated endocytosis could result from subtle rather than dramatic changes in protein levels and/or their activities, it is likely that future studies on adaptive clathrin-mediated endocytosis will be challenging. Most importantly, the studies in the lab need to be confirmed in cells directly derived from human tumours or tumorous tissues.
These recent studies from Professor Schmid and her group suggest that the highly orchestrated process of clathrin-mediated endocytosis, regulated by dynamin, can be exploited by cancer cells to promote their survival. Her future exploration into adaptations of endocytosis used by cancer cells may lead to novel therapeutic targets that combat cancer and reduce the spread of tumours, while shedding light on the remaining mysteries of intracellular trafficking.
Meet the researcher

Professor Sandra Schmid
Department of Cell Biology
University of Texas Southwestern Medical Center
Dallas, TX
USA
Born in Canada, Professor Sandra Schmid moved to the US in 1980 to complete her PhD at Stanford University. She was a postdoctoral fellow at Yale University before taking up a position at The Scripps Research Institute (TSRI) as an Assistant Professor in 1988. From 2000–2012, she served as Professor and Chairman at TSRI before being recruited to the University of Texas Southwestern (UTSW) in 2012, where she currently serves as Chair of the Cell Biology Department and holds the Cecil Green Distinguished Professor of Cellular and Molecular Biology. She has published over 150 papers in peer-reviewed journals, which together have been cited more than 19,000 times. Her research focuses on the major pathway by which cells take up nutrients and signalling hormones, called clathrin-mediated endocytosis. This involves studying the cellular machinery used to form and package small vesicles containing important cellular cargo. A leader in the scientific community, she has won numerous awards, including a National Institutes of Health MERIT Award, as well as leadership and investigator awards from the American Society for Biochemistry and Molecular Biology, the American Society for Cell Biology and the Biophysics Society, reflecting the interdisciplinary nature of her work. She was elected to the American Academy of Arts and Sciences in 2015.
CONTACT
E: Sandra.Schmid@UTSouthwestern.edu
T: (+1) 214 648 3941
W: http://www.utsouthwestern.edu/labs/schmid/
KEY COLLABORATORS
Gaudenz Danuser, Professor and Chair of Bioinformatics (UTSW)
Marcel Mettlen, Assistant Professor (UTSW)
Vadim Frolov, Professor, (Biophysics Institute, Bilbao, Spain)
FUNDING
National Institutes of Health (NIH) – NIGMS and NIMH
Welch Foundation
REFERENCES
PH Chen, N Bendris, YJ Hsiao, CR Reis, M Mettlen, HY Chen, SL Yu and SL Schmid, Crosstalk between CLCb/Dyn1-Mediated Adaptive Clathrin-Mediated Endocytosis and Epidermal Growth Factor Receptor Signaling Increases Metastasis, Developmental Cell, 2017, 40, 278–288, e275.
CR Reis, PH Chen, N Bendris and SL Schmid, TRAIL-death receptor endocytosis and apoptosis are selectively regulated by dynamin-1 activation, Proceedings of the National Academy of Sciences USA, 2017, 114, 504–509.
CR Reis, PH Chen, S Srinivasan, F Aguet, M Mettlen and SL Schmid, Crosstalk between Akt/GSK3beta signaling and dynamin-1 regulates clathrin-mediated endocytosis, The EMBO Journal, 2015, 34, 2132–2146.
SL Schmid, Reciprocal regulation of signaling and endocytosis: Implications for the evolving cancer cell, The Journal of Cell Biology, 2017, 216, 2623–2632.
SL Schmid and VA Frolov, Dynamin: functional design of a membrane fission catalyst, Annual Review of Cell and Developmental Biology, 2011, 27, 79–105.



