Professor Brian Schaefer – Cellular Polka and Immune Cell Signalling
Immunology remains an important branch of medical and biological sciences, providing us with protection against infection and disease. Professor Brian Schaefer of the Uniformed Services University, Bethesda, has dedicated his research to elucidating the molecular mechanisms of immune cell signalling, with the hope of discovering potential therapeutic targets for immunological drugs – to ultimately treat human diseases including autoimmunity, graft rejection and cancers.
A Century of Immunology
Impaired function of the immune system is known to be responsible for a vast number of medical conditions, such as cancers, inflammatory disorders, allergies and autoimmune diseases, where the immune system attacks the body’s own tissues instead of fighting infections. Since 1908, when Russian biologist Ilya Ilyich Mechnikov and German biologist Paul Ehrlich were awarded a Nobel prize for their studies of the immune system, research in the field of immunology has advanced and developed to an incredible degree. To this day, a significant number of researchers and groups dedicate their life’s work to new discoveries in immune system biology, with the aim to help treat or prevent human disease.
Immunology research has provided us with many revolutionary discoveries, from identifying the different cellular components of the immune system to developing vaccinations and therapeutic strategies to combat many devastating diseases, including smallpox, polio and measles. Despite these incredible advancements made in our understanding of immune system biology, gaps in our knowledge of immune function and a lack of therapies for many immune system disorders continue to be major world-wide contributors to deadly disease.
The Finer Details of Immunology
From the earliest accounts of immunology in 1549, with the inoculation of smallpox, our understanding of the immune system has advanced significantly. During the 20th century, researchers identified the various components of the immune system and started to unravel the key mechanisms that allow us to defend ourselves from harmful invading organisms.
White blood cells are key players in immune system defence and can be divided into B cells and T cells that engage in two different kinds of immune response. B cells produce antibodies. These are large proteins that circulate in the blood and fight against foreign bacteria and viruses, by binding to specific proteins found on their surface (antigens). Because of the vast diversity of B cells, antibodies can be generated to bind nearly any antigen.
‘Our overall goal is to define how the transmission of cellular signals is regulated by the formation of organised structures, called “signalosomes”, within the cell.’
This ability to determine friend from foe and recognise what is part of yourself and what has come from elsewhere is key to targeting harmful invading bacteria and viruses for destruction. The binding of antibodies blocks or impairs the ability of the pathogen to function and can target these invaders for destruction by other cells in the immune system.
Another of the vital components of our immune system are the T cells. T cells recognise foreign antigens on the surface of host cells that have been invaded and destroy the infected cells. T cells have antigen receptors on their cell surface responsible for recognising and binding fragments of antigens – proteins the body recognises as foreign. This antigen receptor, called the T cell receptor, activates T cells through a biochemical signal that provokes them to attack.
Professor Brian Schaefer began his career in this field as a postdoc, working with Drs Philippa Marrack and John Kappler, where he started to investigate the mechanisms of immune cell signalling through the T cell receptor. He has since deepened his research within this area, and he has expanded his research to additional areas, including host defences, cellular mechanisms of disease, and cell injury and repair.
Professor Schaefer continued his work in this field when he joined the Uniformed Services University, where he dedicated his attention to defining the molecular mechanisms of T cell receptor signalling to a transcription factor called Nuclear Factor kappa-B (NF-κB) – a protein that controls gene expression. NF-κB is of central importance in T cell biology because it turns ‘on’ the expression of many genes involved in the changes that occur to T cells when they are activated during an immune response.
When a T cell receptor recognises and binds to an antigen fragment, it initiates a series of signals that ultimately activate NF-κB leading to T cell activation and an appropriate immune response. Professor Schaefer’s team has revealed the nature of NF-κB activation through the T cell receptor, discovering that activating NF-κB occurs in a digital, switch-like manner.
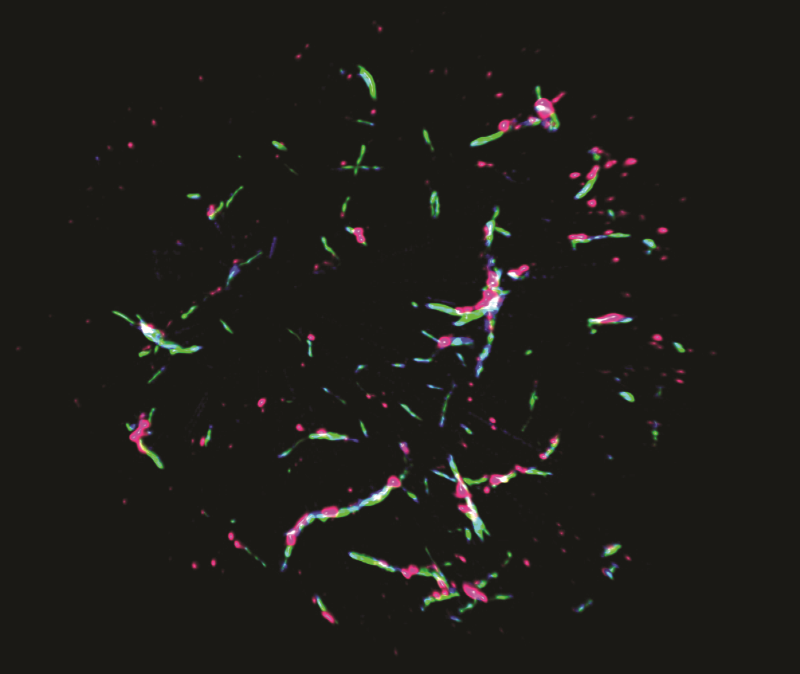
Super-resolution microscopy reveals the components of the POLKADOTS signalosome, showing that Bcl10 (blue), Malt1 (green) and the activated IKK complex (pink) are all components of POLKADOTS filaments.
T Cell Receptor Signalling
The signalling cascade involved in T cell activation through the T cell receptor is extremely complex, with many different components influencing the process. Professor Schaefer’s team has investigated the many components of the signalling cascade that ultimately drive NF-κB activation and the consequent activation of T cells and their rapid multiplication after they detect a foreign protein that is important for an appropriate immune response.
NF-κB is of particular importance, as it stimulates the generation of a large number of other molecules that play a role in the response. The adaptor protein, Bcl10, also plays a large role in transmitting signals from the T cell receptor to NF-κB. Without the Bcl10 protein, T cells are unable to multiply and develop after T cell receptors engage with foreign antigens.
At the same time that T cells activate NF-κB, Bcl10 is degraded and removed. This suggests that this degradation of Bcl10 may be a regulatory mechanism that limits T cell receptor signalling to NF-κB, acting as a brake on the immune response. Although previously, the existence of such a mechanism was controversial, more recent studies by Professor Schaefer’s group and others have confirmed that T cell receptor stimulation induces this intracellular degradation, precisely targeting and delivering components of the cell such as Bcl10 for ultimate destruction. This work revealed that the process of Bcl10 degradation regulates functions that control T cell survival and multiplication during an immune response.
Professor Schaefer and his colleagues explored further the link between T cell receptor-induced destruction of Bcl10 within the cell and the modulation of activated T cell responses. The team revealed that as T cells target the Bcl10 protein for destruction, this process also reduces the ability of T cell receptors to activate NF-κB, and limits the NF-κB induced immune response. They suggest that this mechanism modulates antigen receptor signalling and propose that this process may protect T cells from the adverse consequences of unrestrained NF-κB activation and an overactive immune system, such as cellular aging.
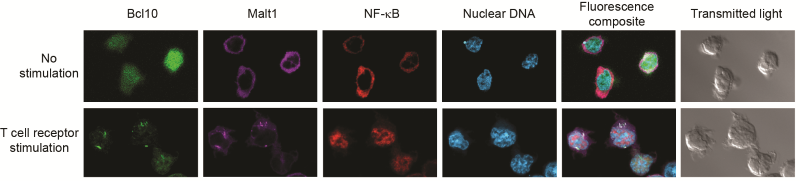
T cell receptor stimulation causes POLKADOTS formation and NF-ĸB activation. Confocal microscopy shows that delivery of an activating signal to the T cell receptor causes Bcl10 and Malt1 to move into filamentous POLKADOTS, NF-ĸB then moves from the cytoplasm into the nucleus – identified by staining nuclear DNA.
Building the Structures for Signalling
Professor Schaefer states that, ‘our overall goal is to define how the transmission of cellular signals is regulated by the formation of organised structures, called “signalosomes”, within the cell.’ Early T cell receptor signals involve the formation of a CBM (Carma1-Bcl10-Malt1) complex, containing the Bcl10 adaptor protein, Carma1 (a larger adaptor protein) and Malt1 (an enzyme that breaks down proteins). Assembly of this complex triggers activation of another signalling complex, IKK, the key regulator of NF-κB activation. After being activated by IKK, NF-κB is able to move into the nucleus of the cell to activate target genes.
Professor Schaefer and his colleagues noted that there was a lack of understanding of how these proteins interact, and conducted research to elucidate the signalling mechanisms involved in this process. In their early work, Professor Schaefer’s group had identified T cell receptor-induced formation of signalling clusters of Bcl10 and Malt1, which make up a ‘signalosome,’ which they named POLKADOTS, an acronym describing the dotted appearance of these structures within a stimulated T cell.
The team found that the abundance of these signalling clusters is highly correlated with how much NF-κB moves into the nucleus, suggesting that the POLKADOTS structure plays a role in activating NF-κB. Professor Schaefer remarks that, ‘now that we know something about what this signalling machine does, we are trying to understand exactly how it works. This information could ultimately lead to new drugs for altering immune responses to treat disease.’
The Bcl10 protein’s ability to form filaments is correlated with its ability to activate NF-κB. Professor Schaefer’s research group found that the active form of IKK is located only at the POLKADOTS signalosome, providing compelling evidence that this filamentous structure controls NF-κB activation.
Furthermore, when these filaments cluster around pre-existing aggregates of a protein called p62, POLKADOT structures form. They confirmed this finding by disrupting p62, which stopped POLKADOTS from forming and blocked NF-κB from being activated. The team showed that in mice genetically modified to remove p62 there was a change in T cell receptor-dependent IKK and NF-κB activation and T cell activation during an immune response.
Together, their work has shown that p62, Bcl10, and Malt1 form POLKADOTS – the ‘signalosome’ that directs the activation of NF-κB, ultimately controlling the rate at which target genes are turned ‘on’ or activated. This makes POLKADOTS an attractive target for developing immunomodulatory drugs. For example, drugs inhibiting formation of POLKADOTS may have applications in the treatment of autoimmune diseases or other conditions that are characterised by an undesired overactivation of T cells.
‘We are using unique cutting-edge microscopes to make high-resolution videos of the POLKADOTS signalosome in living T cells. The resulting movies will document the changes in this signalosome over time, enabling us to better understand how this complex molecular machine performs its functions within the cell.’
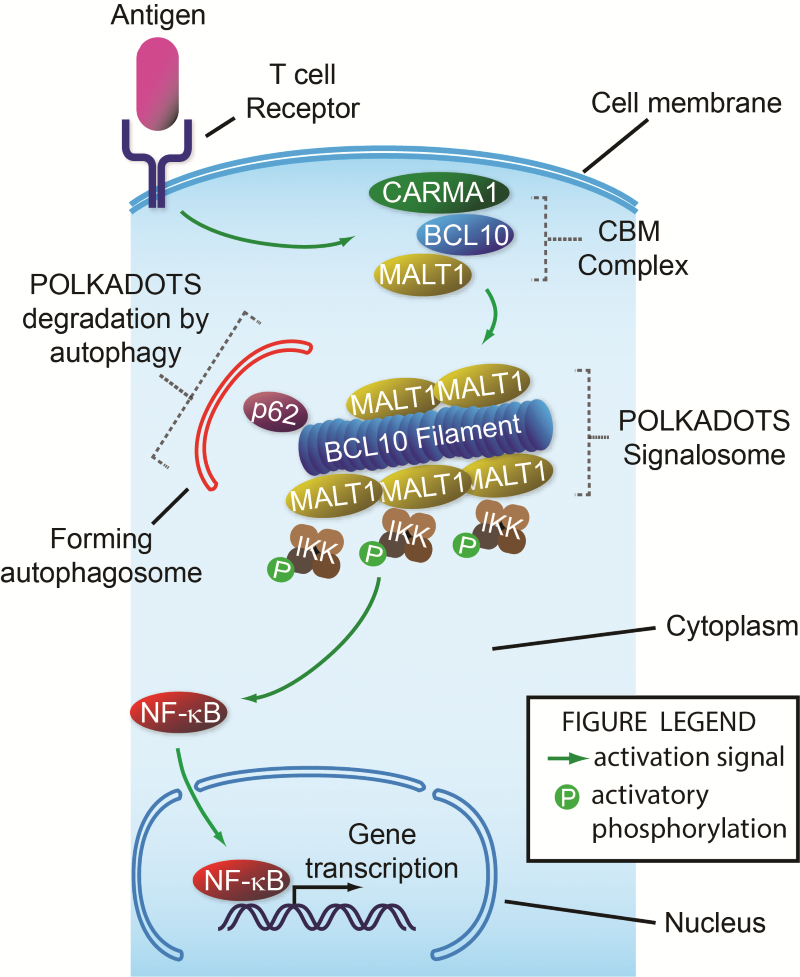
Steps in signalling from the T cell receptor to NF-ĸB. When a T cell receptor binds its target, a signal is sent, causing assembly of the CBM complex just inside the cell membrane. Next, Bcl10 interacts with p62, forming the filament-shaped POLKADOTS signalosome that contains Malt1 and IKK. This causes IKK to become activated (P-IKK). P-IKK then transmits a signal causing NF-ĸB to move from the cell cytoplasm into the nucleus, where it turns on many genes required for T cell function. At the same time this activation signal is being turned on by the T cell receptor, it is also being partly turned off by autophagy of the POLKADOTS filaments, a destruction process directed by p62 that destroys the Bcl10 protein, reducing activation of NF-ĸB.
Looking to the Future
In order for Professor Schaefer’s group to reach their goal of identifying specific targets for pharmacological drugs to modify immune responses, it is important to define the signalling mechanisms in molecular detail. Professor Schaefer’s team is engaged in studies to elucidate the molecular mechanisms by which the POLKADOTS ‘signalosome’ precisely controls the activation of NF-κB.
Professor Schaefer reveals that, ‘our approach employs several different technical methods, including biochemistry, molecular biology, microscopy, and, most recently, mathematical analyses and modelling with our collaborator, Professor Wolfgang Losert,’ who is working with the team to develop algorithms for analysing patterns of protein distribution.
To better understand how T cells turn off the POLKADOTS ‘signalosome,’ collaborations have also been established with Professors You-Wen He at Duke University School of Medicine and Thomas Conrads at Inova Schar Cancer Institute. Indeed, the majority of Professor Schaefer’s work is now highly collaborative, as he has found that joining forces with other investigators with complementary expertise, ‘greatly increases the breadth of scientific and technical approaches, facilitating a much deeper mechanistic understanding of our scientific questions than could be achieved by my group working in isolation.’
Professor Schaefer explains that, ‘we are completing a study demonstrating that the composition of the POLKADOTS signalosome changes substantially over time, and that it is actually transported and consolidated at a specific location within the cell. These movements are connected to a change in function of the signalosome.’ Discovering the molecular mechanisms by which the POLKADOTS ‘signalosome’ regulates T cell receptor activation of NF-κB will bring the team closer to their goal or identifying targets for novel immunomodulatory drugs.
‘We are also in the midst of an exciting collaboration with the Advanced Imaging Center at the Howard Hughes Medical Institute’s Janelia Farm facility,’ Professor Schaefer reports. ‘In this work, we are using unique cutting-edge microscopes to make high-resolution videos of the POLKADOTS signalosome in living T cells. The resulting movies will document the changes in this “signalosome” over time, enabling us to better understand how this complex molecular machine performs its functions within the cell.’ The hope is this information can be used to develop new treatments for a wide array of human diseases, such as cancer, autoimmunity and graft rejection.
Meet the researcher

Professor Brian C. Schaefer
Department of Microbiology and Immunology
Uniformed Services University of the Health Sciences
Bethesda, MD
USA
Brian C. Schaefer is a Professor in the Department of Microbiology and Immunology at the Uniformed Services University (USU) in Bethesda, MD. Professor Schaefer achieved a Bachelor of Science Degree in Biology from the Massachusetts Institute of Technology in Cambridge and continued to work towards his passion at Harvard University where he was awarded his PhD in Immunology in 1995. From 1996 until 2002, Professor Schaefer worked with Drs Philippa Marrack and John Kappler, investigating Immunology and cell signalling. In 2002, he was offered a faculty position at USU, where he continues to focus his research on exploring signal transduction mechanisms of immune cells including host defences, cell mechanisms of disease and cell injury and repair. Professor Schaefer is a Chair of the Faculty Advisory Committee of the USU Biomedical Instrumentation Centre and sits on the Editorial Board of Frontiers in Cell and Developmental Biology. Professor Schaefer has achieved numerous honours and awards for his outstanding contributions to Microbiology and Immunology research including a Kimmel Scholar Award in 2004, a competitive research award from the Dana Foundation Program in Brain and Immuno-imaging in 2005 and the USU Henry Wu Award for excellence in basic science research in 2016.
CONTACT
E: Brian.Schaefer@usuhs.edu
T: (+1) 301 295 3402
W: https://www.usuhs.edu/mic/faculty
KEY COLLABORATORS
Professor Wolfgang Losert, University of Maryland
Professor Arpita Upadhyaya, University of Maryland
Professor You-Wen He, Duke University School of Medicine
Professor Thomas Conrads, Inova Schar Cancer Institute
Professor Christopher Broder, Uniformed Services University
Professor Andrew Snow, Uniformed Services University
Professor Igor Brodsky, University of Pennsylvania
Professor Hao Wu, Harvard University
FUNDING
National Institutes of Health (NIH)
REFERENCES
LM Kingeter, S Paul, SK Maynard, NG Cartwright, and BC Schaefer, Cutting Edge: T cell receptor ligation triggers digital activation of NF-kB, The Journal of Immunology, 2010, 185, 4520–4.
S Paul, AK Kashyap, W Jia, Y-W He and BC Schaefer, Selective Autophagy of the Adaptor Protein Bcl10 Modulates T Cell Receptor Activation of NF-kB, Immunity, 2012, 36, 947–958.
S Paul and BC Schaefer, A new look at antigen receptor signaling to NF-kB, Trends in Immunology, 2013, 34, 269–81.
S Paul, MK Traver, AK Kashyap, MA Washington, JR Latoche and BC Schaefer, T Cell Receptor Signals to NF-kB Are Transmitted by a Cytosolic p62-Bcl10-Malt1-IKK Signalosome, Science Signaling, 2014, 7, ra45.



