Professor Urs Schaltegger – Using Radioisotopes In Volcanic Crystals To Measure The Age Of The Earth
Every child has asked a grandparent: ‘How old are you?’ Often the answer laughingly is: ‘I’m as old as the hills!’ Of course, this is a loose reference to a verse in the Bible in the book of Job: ‘Art thou the first man that was born? Or wast thou made before the hills?’ It follows, naturally, that the next question the child might ask is: ‘Well, then, how old are the hills?’ To answer that requires more than the Bible – we need science. A good place to turn to for that answer is Professor Urs Schaltegger and his colleagues in the University of Geneva’s Department of Earth Sciences. They utilise the modern science of geochronology to pinpoint the exact age of ‘the hills’.
Geochronology is the branch of science dealing with the age of rocks, sediments and fossils by studying chemical or physical properties of the materials themselves, rather than characteristics of the surrounding area. We commonly think of determining the age of a dinosaur bone or bones of an extinct hominid by looking at the layers of sediment in which we find it – the deeper the layer, the older the bones. But geochronologists want to analyse the material itself to determine the age. Analogous to archaeologists’ radiocarbon dating of prehistoric plant and animal material by determining their level of carbon-14, Professor Schaltegger and his group dates rocks and geologic materials by measuring the decay of radioactive uranium into lead.
Estimating the Age of the Earth from Radioactive Decay
For over three centuries, scientists have attempted to determine the age of the Earth. Initially, they studied proxies of the Earth’s age, such as the salinity of the seas. As time passes, more material – including salt – washes into the oceans, making them saltier. If we back calculate from the rate of increase in sea salt, we can estimate how long this has been happening. Clearly this is a bit dicey. When did the first ocean form, anyway? How salty was it to begin with? Was the rate of erosion always the same? Certainly we would like something more accurate. But after the French scientist Henri Becquerel discovered radioactivity in 1896, whole vistas of science opened up. And in 1911 a pioneer in geochronology, British geologist Arthur Holmes, conceived the idea of measuring the decay products of uranium in geologic material to determine the age of the material. Specifically, Holmes looked at the radioactive decay of isotopes of uranium into lead isotopes in geologic specimens and used the known half-life of the different uranium isotopes to calculate the age of the geologic specimen. This half-life countdown of uranium actually includes two nuclear clocks:
Uranium is found in nature in two different isotopic forms, uranium-238 and uranium-235. Uranium-238 naturally decays into lead-206. This decay has a half-life of 4.468 billion years, or, as the scientists say, 4.468 gigaannus (Ga). Uranium-235 decays into lead-207, with a half-life of 704 million years, or 704 megaannus (Ma). Using both of these countdown clocks, scientists like Professor Schaltegger can measure the age of geologic material with very good precision and accuracy, depending upon the concentrations and purity of uranium and lead in the material. Professor Schaltegger and his group at the University of Geneva are learning to read these countdown clocks to more accurately reconstruct the timing of major processes in the geologic history of the Earth.
‘Knowing the time allows us to understand geological processes. With our understanding we are able to link processes in the deep earth to what’s going on at the surface.’

Where in the World is the Uranium?
To be able to accurately use uraniumlead (U-Pb) dating, you first have to have geologic material with appropriate amounts of uranium. Scientists have found that the favourite material is the mineral zircon, a zirconium silicate, or ZrSiO4. This mineral crystallises over time in cooling magma, deep below the Earth’s surface. During these processes, zircon not only incorporates atoms of zirconium and silica, but also impurities – among them uranium. Looking into this at the nanometre scale (a nanometre is a billionth of a meter – 10-9 m) we can see that molecules within the zircon crystal lattice can be replaced by other molecules, thereby including traces of other compounds in the lattice. One of those impurities that perfectly fits into zircon is coffinite, the uranium silicate USiO4. Uranium is therefore found as an impurity, or a socalled ‘trace element’, in our zircon crystal. Importantly, what is not easily included as an impurity in the zircon crystal is lead – it just hasn’t the right size to fit in the zircon lattice. So zircon is an ideal material that forms in the Earth with a little uranium, but almost no lead. This means any lead found in zircon millions of years after its formation wasn’t there to begin with. It must be a daughter product of the radioactive decay of uranium. Scientists like Professor Schaltegger can then use advanced modern technologies of isotope separation, using mass spectrometers, to measure the ratios of uranium isotopes and lead isotopes. Since we know the half-lives the isotopes, we can calculate how long ago this specimen of zircon crystallised. This is the basis for U-Pb dating.
Going back to our sea salt analogy, the erosion rate by which salt is washed into the sea can be thought of as our radioactive decay constant, while the initial salt concentration in seawater is equivalent to the initial lead concentration at the moment of zircon crystallisation.
U-Pb dating techniques can be applied to a large variety of geologic problems where the durations and rates of processes need to be precisely and accurately known. ‘By knowing the process rates, we can conclude on the nature of processes and judge whether models are realistic or not,’ explains Professor Schaltegger.
For example, if you obtain and date samples of zircon from different rocks of an area with magmatism and volcanism, you may be able to reconstruct the fluxes of liquid magma from the Earth’s interior to the surface and this would give us a picture of the dynamics of the magma flow even though it happened millions of years ago. This information could be crucial to estimate the risk of volcanic eruptions in a certain area, or how much a magma would have been capable of forming an ore deposit, for instance one of gold or copper. If we know how old the zircon is in one area – basically, when it initially crystalised from the liquid magma – we can contrast that with the age of zircon at another site. Eventually, we might be able to draw a picture of how magmatism, volcanism, rock uplift, erosion, and even topography, evolved in response to plate tectonics.
On a planetary scale, being able to date the oldest zircon crystals of our planet allows us to measure when the earliest materials condensed when the Earth started to cool.
The long half-life of uranium-238 particularly allows us to look back literally hundreds of millions to billions of years. For Professor Schaltegger, some of the interesting aspects of our Earth’s history are periods of profound disturbance of life, climate and environment during the history of the Earth, popularly termed mass extinctions. ‘The geological record often does not allow us to prove the causality between two processes directly, a prime example being mass extinctions and volcanism,’ he tells us, ‘but we need to make the detour via age determination. Since geology is a “historical” science that tells the story of the earth, time is essential.’

Mass Extinctions are Unusually Hot Topics!
Mass extinctions are intervals of widespread and rapid decrease in life forms on Earth. Since microscopic life is not easy to see in fossils and other geologic material, we usually talk about a decrease in multicellular life, namely animals and vegetation. Mass extinctions in Earth’s history were associated with massive volcanic activity. Most people are familiar with the Cretaceous-Paleogene extinction of the dinosaurs 66 Ma ago, presumably due to the onset of a several hundred thousand year-long period of intense volcanism in the present-day area of the Deccan traps in India. This is the event that is thought to have killed off roughly three-quarters of animal and plant life on Earth. It seems that the environmentally rough conditions additionally degraded with the impact of the 10 km diameter asteroid or comet that created the Chicxulub crater in the Yucatan peninsula in Mexico.
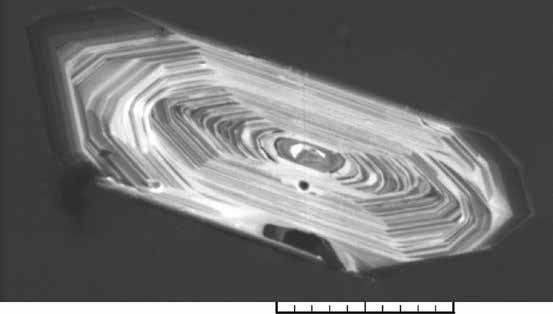
The temporal coincidence between mass extinctions and periods of massive volcanic eruptions serves as an argument for linking global climatic and environmental catastrophe with the sudden injection of large volumes of gases – such as carbon dioxide, methane or sulphur dioxide – into the stratosphere. These gases are either directly released from decompressing magma when it flows into the upper crust and flows onto the terrestrial surface, or they may be derived from extreme heat – sometimes over 1000°C – acting upon organic-rich sedimentary rocks closer to the surface. As such, these events resulting from volcanism are ideal targets for Professor Schaltegger’s U-Pb dating strategy. Volcanoes means magma and lava, containing tiny crystals of zircon. Zircon means we have a countdown clock for dating purposes. In fact, Professor Schaltegger and his team, along with collaborators in Europe and overseas, are studying important mass extinction intervals in the history of the Earth, such as the boundary between Cathodoluminescence images of a zircon crystal that shows how this mineral is growing in a magmatic liquid by adding layers of crystallised material. The difference in greyscale is indicating different chemical composition (black = much uranium; light = much Yttrium) the Permian and Triassic 252 Ma ago, the Triassic–Jurassic extinction event, marking the boundary between the Triassic and Jurassic periods 201.3 Ma ago, and the Pliensbachian-Toarcian event – a global crisis reflected by oxygen depletion in global ocean water – marking the end of the Pliensbachian stage and the start of the Toarcian stage of the Early Jurassic period, 183 Ma ago. At the end of the Permian, some 96% of all marine species and 70% of vertebrate terrestrial species became extinct, which makes the Permian-Triassic extinction the most severe life crisis on our Earth. The Triassic-Jurassic event resulted in the extinction of roughly half of existing species and allowed the fabled rise of the dinosaurs. The Pliensbachian-Toarcian event is associated with global extinction of many groups of invertebrates such as many of the existing ammonites at that time. What Professor Schaltegger and colleagues did was compare and combine biostratigraphy data and chemical indicators of climate and biodiversity change, such as isotopic composition of carbon and oxygen, with high precision U-Pb zircon dates from the three boundaries – Permian-Triassic, Triassic- Jurassic and Pliensbachian–Toarcian.
Recent results from the research carried out by a group of scientists including Professor Schaltegger suggest that these events were associated with rapid change from an initial cool period to hotter greenhouse conditions. They reason that this transition resulted from changing gas species emitted during the progressive thermal erosion of Earth’s crust by plume activity. Their model is that initial gas emission was dominated by sulfur liberated from sulfide-bearing rock formations within the upper Earths’ mantle before carbon dioxide became the dominant gas. The important conclusion from the team’s analysis is that it was volcanic activity that caused massive shifts in life on Earth. Much of their reasoning is derived from the measurement of isotopes in crystals from volcanic rocks. In other words, they are explaining what happened to life on Earth by reconstructing the story that rocks on Earth can tell us.
According to Professor Schaltegger, he was always interested in the aspect of time in geology and was fascinated by the possibility to quantify it. ‘As a second-year student I started to work in the isotope lab at University of Bern,’ he tells us. ‘Time is the basis for understanding geology, and I find fascinating that you can reconstruct and tell a story about the Earth – a Fairy Tale about the Earth.’ The group constantly tries to improve the reliability of isotopic dates. As Professor Schaltegger explains: ‘Data have to be precise, reproducible and comparable between labs and over time. Thus, coordination with laboratories at other centres is important.’ Professor Schaltegger’s group belongs to the EARTHTIME consortium, an international scientific initiative aimed at accurately determining the history of the Earth through the integration of high-precision geochronology and quantitative study of geologic strata. Their next steps will be to establish the possibility of extracting parts of zircon grains and analysing their chemical and isotopic composition, and thus their age, separately. This can give them greater accuracy in their determination of the age of a particular piece of zircon. ‘We need to advance our understanding of how changes in zircon and other datable minerals react to temperature, pressure, circulating fluids and other external forces,’ says Professor Schaltegger. In other words, they want to understand which processes they are actually dating – just knowing the date is only a start.
Dating geologic specimens opens the door to understanding what actually happened back there in time. Speaking geologically, for instance, give answers to questions like how and in what timescales are ore deposits formed? How are the processes deep under the Earth linked to active volcanism? How do diamond-bearing rocks come up from 150 km to 20 km depth and how long does that take? Professor Schaltegger hopes to answer these and many more questions about the Earth and its history by applying his scientific knowledge and techniques to finding out how old the hills actually are.
Meet the researcher

Head of the Isotope Geochemistry Group Department of Earth Sciences
University of Geneva
Switzerland
Professor Urs Schaltegger obtained his PhD in 1989 from the University of Berne, Switzerland. From 1989 into 1996, he held several postdoctoral research fellowships mainly funded by the Swiss National Science Foundation at various centres, including the Royal Ontario Museum’s Geochronology Laboratory, the Research School of Earth Sciences at the Australian National University, the CNRS Centre for Surface Geochemistry in Strasbourg, France, and the ETH Zürich, in Zürich, Switzerland, where he held a Senior Researcher/Lecturer position from 1997–2001. In 2001, he joined the faculty at the University of Genève, where he teaches courses in mineralogy, geology and isotope geochemistry at both the undergraduate and graduate levels, as well as supervising graduate and post-graduate members of the Isotope geochemistry group.
Professor Schaltegger’s research interests include developing state-ofthe art analytical techniques for high-precision Uranium-Lead dating, studying timescales for the formation of Large Igneous Provinces and their intercalibration with periods of mass extinction, calibration of geological time scale by Uranium-Lead dating of zircon found in volcanic ash beds, and timing and tempo of magmatism in the Earth’s crust. He is developing concepts to understand how the mineral zircon and its Uranium-Lead isotopic system react to changes in the ambient geological environment.
CONTACT
T: (+41) 22 379 66 38
E: urs.schaltegger@unige.ch
W: http://cms.unige.ch/sciences/terre/people/personal_pages/UrsSchaltegger/UrsSchaltegger
KEY COLLABORATORS
Pavel Holoborodko, Multiprecision Computing toolbox, Kanagawa, Japan.
Alexandre I. Fedoseyev, Computational Fluid Dynamics Corp., Huntsville, Alabama, USA
Juergen Gieser, Ruhr Universitaet, Bochum, Germany
Levan Ling, Hong Kong Baptist University, Hong Kong
Yiu-Chiug Hon, City University of Hong Kong, Hong Kong
Antonio Fererra, University of Porto, Oporto, Portugal
Nicolas A. Libre, Missouri Science and Technical University, Missouri, USA
Anita Wong, Open University of Hong Kong, Hong Kong
Efim A. Galperin, University of Quebec at Montreal, Montreal, Quebec, Canada
Athena Markogolu, University of Portsmouth, Portsmouth, UK
Scott Sarra, Marshall University, Hunington, West Virginia, USA
FUNDING
Swiss National Science Foundation
European Commission 7th Framework Programme for Research
European Commission Horizon 2020 Programme
REFERENCES
J Guex, S Pilet, O Müntener, A Bartolini, J Spangenberg, B Schoene, B Sell and U Schaltegger, Thermal erosion of cratonic lithosphere as a potential trigger for mass-extinction, Scientific Reports, 2016, 6, 23168.
U Schaltegger, A Schmitt and M Horstwood, U-Th-Pb zircon geochronology by ID-TIMS, SIMS and laser ablation ICP-MS: recipes, interpretations and opportunities, Chemical Geology, 2015, 402, 89-110
L Caricchi, G Simpson and U Schaltegger, Zircons reveal magma fluxes in the Earth’s crust, Nature, 2014, 511, 457.
JF Wotzlaw, U Schaltegger, DA Frick, MA Dungan, A Gerdes and D Günther, Tracking the evolution of large-volume silicic magma reservoirs from assembly to supereruption, Geology, 2013, 41, 867–870.
J Leuthold, O Müntener, LP Baumgartner, B Putlitz, M Ovtcharova and U Schaltegger, Time resolved construction of a bimodal laccolith (Torres del Paine, Patagonia), Earth and Planetary Science Letters, 2012, 325–326, 85–92.
B Schoene, J Guex, A Bartolini, U Schaltegger and TJ Blackburn, Correlating the end-Triassic mass extinction and flood basalt volcanism at the 100 ka level, Geology, 2010, 38, 387–390.



